(The Daily Sceptic) For six months, the MHRA and other national regulators have been sitting on a Pfizer report about Covid vaccine safety. Worryingly, the abstract which I have just found online doesn’t look good at all:
- the vaccinated cohort have at least 23-40% higher risk of some heart-related conditions; and
- the risk is higher than in Pfizer’s previous report (i.e., it is increasing over time since vaccination).
The report in question is Pfizer’s report C4591021 ‘Interim Report 5’ dated March 12th 2024. It is a Post Authorisation Safety Study (PASS) of Pfizer’s Covid vaccine. In summary, national regulators routinely require pharmaceutical manufacturers to conduct PASS studies as a condition of authorisation of most new medicines. The regulators provide data to the manufacturer covering millions of patients registered in national healthcare systems. The manufacturer then conducts analysis to determine whether the medicine has increased the risk of specified health conditions.
I have previously written a couple of articles about Covid vaccine PASS studies. First, in October 2023, to raise awareness of the studies and the fact that most of them were not being published. Second, in January 2024, to report that I had obtained copies of PASS studies by Pfizer, Moderna and AstraZeneca via a Freedom of Information request to the MHRA. In the second article I picked out three health conditions (arrhythmia, heart failure and acute coronary artery disease) from Pfizer’s ‘Interim Report 4’ where there was a higher incident rate in the vaccinated cohort.
Knowing that Pfizer had completed its ‘Interim Report 5’ in March 2024, in April I submitted FOI 24/075 to MHRA asking for a copy. MHRA applied a Section 22 Exemption: “information intended for future publication.” This seemed very odd given that it had sent me previous ones only three months before. However, helpfully, it stated that it “will be published in the fourth quarter of 2024”.
So in late August, I submitted another FOI (24/475) to check that this was still MHRA’s intention. Imagine my surprise when it backtracked: “We cannot confirm whether the Pfizer C4591021 Interim Study Report 5 prior to December 31st 2024 is still due to be published. We have contacted the company, who have informed us that the final report is due for submission at the end of 2024 and plans for publication will be decided at this point.” I read that as: “We’re worried about the results in Interim Report 5, so we’ve decided to wait for Pfizer’s Final Report before deciding if and when to publish either of them.”
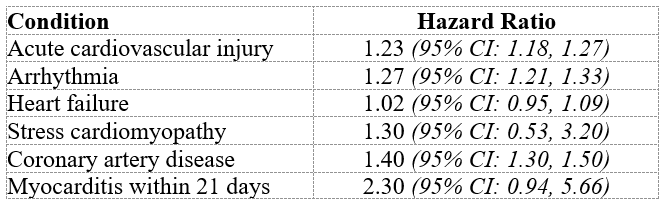
Imagine my further surprise when I just found an abstract of Pfizer’s ‘Interim Report 5’ online. As I said at the start, it doesn’t look good. Here are the first six conditions mentioned in the abstract: