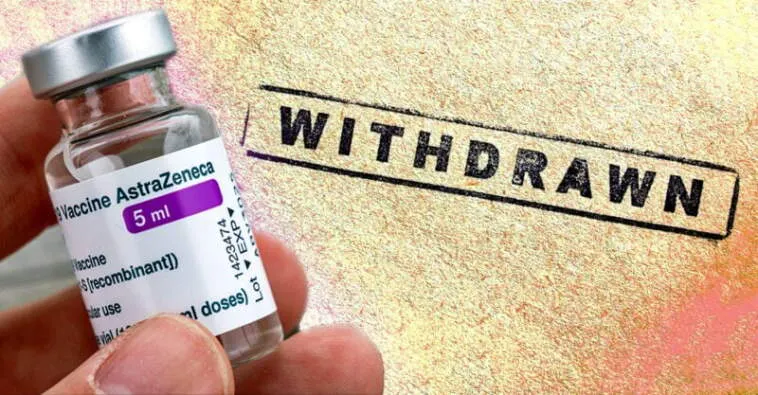
(The Defender) AstraZeneca on Tuesday said it has begun a worldwide withdrawal of its COVID-19 vaccine, Vaxzevria, Reuters reported.
In a statement, the company said it is withdrawing the drug because there is a “surplus of available updated vaccines” adapted for new variants of the virus. The new drugs created a decline in demand for Vaxzevria, it said, which is no longer manufactured or supplied.
The announcement comes just months after the drugmaker admitted in legal documents submitted to the U.K. High Court that the drug “can, in very rare cases, cause TTS.”
Thrombosis with thrombocytopenia syndrome (TTS) — also referred to as vaccine-induced thrombotic thrombocytopenia — is linked to the AstraZeneca and Johnson & Johnson COVID-19 vaccines. The condition causes the body to produce blood clots, which can be life-threatening.
AstraZeneca is a defendant in a class-action lawsuit in the U.K. by 51 claimants alleging serious injuries from the drug. Twelve of the claimants are acting on behalf of a loved one who allegedly died from vaccine-induced blood-clotting issues.
On March 5, the pharmaceutical giant voluntarily withdrew its European Union marketing authorization required for marketing a drug in the EU. The European Medicines Agency, on Tuesday, issued a notice that Vaxzevria is no longer authorized for use.
AstraZeneca will make similar withdrawal applications in the coming months in the U.K. and other countries that have approved the vaccine, The Telegraph reported.
AstraZeneca said the decision to withdraw the drug was not linked to its admission about TTS and that the timing was coincidental.
In a statement the company said:
“We are incredibly proud of the role Vaxzevria played in ending the global pandemic. According to independent estimates, over 6.5 million lives were saved in the first year of use alone and over three billion doses were supplied globally.
“Our efforts have been recognised by governments around the world and are widely regarded as being a critical component of ending the global pandemic. …
“We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
Vaxzevria, also marketed as Covishield, uses viral vector technology, unlike the Moderna and Pfizer COVID-19 shots that use mRNA technology.
The AstraZeneca vaccine is made from another virus of the adenovirus family. The virus is modified to contain the gene for making a protein from SARS-CoV-2, according to The Guardian.
The vaccine was developed by Oxford University and AstraZeneca.
The drug was authorized for use in the U.K., Europe and Australia. It was approved for an emergency use listing by the World Health Organization and delivered to low- and middle-income countries through COVAX, funded by the Bill & Melinda Gates Foundation.
Gates heavily invested in the vaccine.
By late 2021, the AstraZeneca vaccine was largely replaced by the Pfizer and Moderna jabs in the EU, but as of January 2022, 2.5 billion doses of the drug had been administered worldwide.
The drug was never authorized in the U.S.