From NYpost.com…
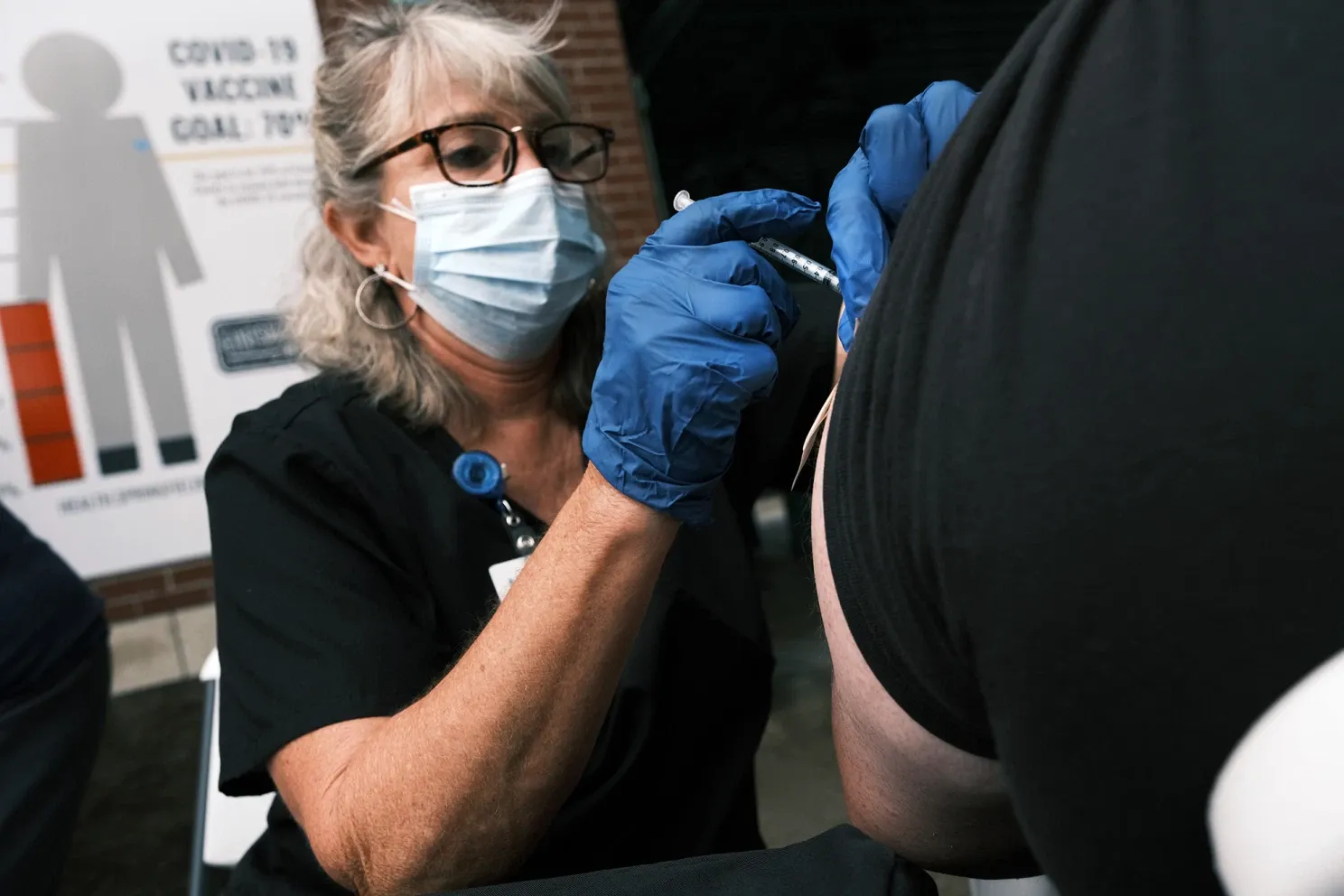
The Food and Drug Administration has approved an additional booster vaccination dose for those with weak immune systems or who have received organ transplants, it announced on Thursday.
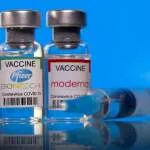
The FDA said that a third dose of the Pfizer or Moderna vaccine may increase protection in those that are “immunocompromised” or have undergone a solid-organ transplant.
Previously only two doses have been authorized by the FDA for emergency use in patients 12 years and older.
“The country has entered yet another wave of the COVID-19 pandemic, and the FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease. After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines,” said Acting FDA Commissioner Janet Woodcock in a statement.